Abstract
Background
Tregs play an important role in the establishment and maintenance of immune tolerance during pregnancy and they are expanded early after embryo implantation. Decreased frequency and function of Tregs in women suffering from recurrent spontaneous abortions (RSA) has been reported in literature. Signal transducer and activator of transcription (STAT) proteins play a prominent role in the epigenetic control of Treg differentiation. Deficiency of STAT5 leads to loss of Foxp3+ T regulatory cells (Tregs) and inability to induce Tregs in vitro whereas constitutive activation of STAT5β enforces Foxp3 positive Treg development. Aberrant immune signaling via STATs is involved in disease pathobiology in numerous autoimmune conditions, therefore explored the STAT signaling biosignature of Tregs by single-cell phospho-specific flow cytometry in women with RSA before and after treatment with lymphocyte immunotherapy (LIT).
Materials and methods
Peripheral blood mononuclear cells from 31 women with either RSA (n=23) or with infertility and ≥2 unsuccessful IVF (n=8) and 10 age-matched females without RSA who had at least one successful pregnancy, were obtained before and after LIT. Basal levels of phospho-STAT1, STAT3 and STAT5 and potentiated, i.e. target/stimuli, nodes (IFNα/pSTAT1, IFNα/pSTAT5, IL-6/pSTAT1, IL-6/pSTAT3 and IL-2/pSTAT5) were measured by phospho-specific flow cytometry. Treg signaling profiles were grouped by using unsupervised hierarchical clustering and correlated with clinical parameters by using χ2 or Fisher Exact tests. Associations of single signaling nodes with the aforementioned parameters were performed by Mann-Whitney and Wilcoxon signed-rank tests as appropriate.
Results
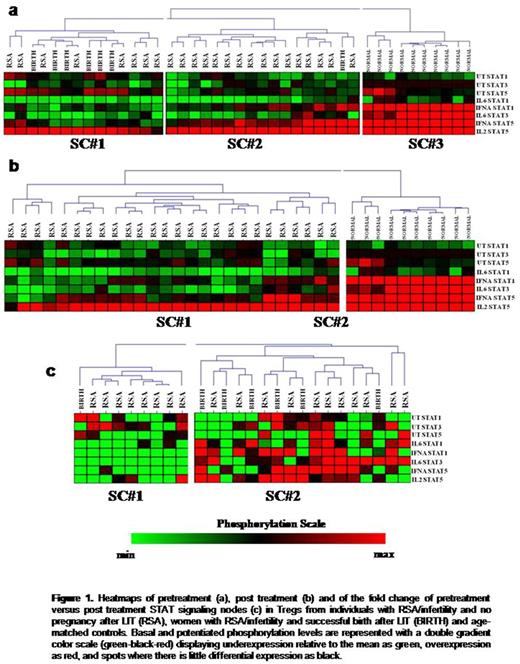
No differences were observed between women with RSA and age matched women without RSA concerning the pretreatment and post treatment levels of Tregs. By contrast, clustering of pretreatment signaling nodes identified three signaling clusters (SC, figure 1a). SC#3 was enriched in women without RSA (p<0.001) and characterized by high potentiated responses of STATs, whereas women with RSA segregated in SC#1 and SC#2 which were characterized by blunted STAT responses in cytokine stimulation. Of note, all women with successful pregnancy after LIT (n=7) were clustered in SC#1 (p=0.028) which was characterized by high basal levels of pSTAT1 and pSTAT5 but weak potentiated responses of all nodes. Also, Tregs in women with a successful pregnancy after LIT displayed a significantly decreased response of pSTAT5 to IFNα (p= 0.05) and increased basal pSTAT5 levels (p=0.047), compared to women who failed LIT. Τhe post-treatment STAT signaling biosignature remained abnormal in women with RSA (p<0.001, figure 1b). However, compared to women with at least one miscarriage (n=23), women with infertility and unsuccessful IVFs displayed decreased post treatment basal levels of pSTAT1 (p=0.004) and pSTAT3 (p=0.01) and increased responses of pSTAT1 (p=0.047) and pSTAT3 (p=0.047) to IL6. Also, clustering the fold change of pre versus post-treatment STAT signaling profiles in Tregs revealed two signaling clusters. The #SC2 was marginally enriched in women with successful pregnancy after LIT (p=0.1) and was characterized by upregulation of all potentiated nodes after treatment (figure 1c).
Collectively, we show for the first time that Tregs in women with RSA bear a pathological STAT biosignature which correlates with LIT outcome. Moreover, LIT can potentially induce a beneficial modulation of STAT signaling by upregulating Treg responsiveness to cytokines. Our work provides novel biological data on the mechanisms of immune tolerance in pregnancy and may point to therapies that can overcome pregnancy loss by modifying cell and cytokine responses.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.